Gas Expansion into a Vacuum
Environment
Introduction
This
animation considers the energy and pressure of a gas that is expanding due to
the motion of one of the walls of its container. For this case I will assume that the wall is
moving uniformly so as to increase the volume accessible to the gas and that
the wall does not experience any retarding force because of any external gas
pressure. This is in some ways more
complicated than the usual gas physics situations where, for example, the gas
expands while the pressure remain constant.
Figures
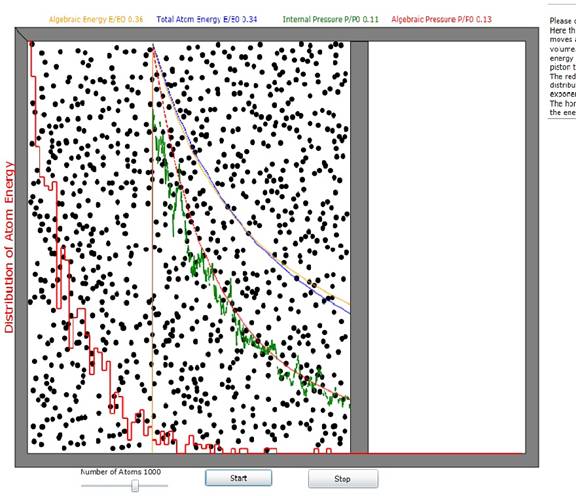
Figure 1: Gas expansion apparatus diagram. The moving wall (piston) starts at the
location of the vertical orange line and moves to the right end of the
chamber. Energy and pressure are plotted
in blue and green, respectively.
Physics and Math
Since the
wall moves away from the occupied volume, the gas atoms lose momentum and kinetic
energy when hitting it. As a result of
the energy reduction they impact all of the walls with less momentum and the
pressure is reduced.
In the reference frame of the piston, the momentum change
can be written:
|
|
|
(1.1)
|
where the prime on pp indicates the value of
momentum after the collision.
In the laboratory reference frame:
|
|
|
(1.2)
|
which results in the following energy change:
|
|
|
(1.3)
|
Obviously vx>vp if the atom is to hit
the piston. When vx=vp
+ δvx
then becomes
|
|
|
(1.4)
|
regardless of the magnitude of δvx.
For an atomic area of xp*h, where h is the piston
height, the number of piston hits per second expected is
|
|
|
(1.5)
|
where xp is the length of the cylinder and h is
the height of the piston.
Thus, combining equations (1.4)
and (1.5),
the expected energy loss per second is
|
|
|
(1.6)
|
When vx is about equal to vp then vx
doesn't change much nor does energy. Now
we must consider another question: how to compute a meaningful value for <vx>
where only
<vx> is greater than vp is
meaningful. That involves integrals over
the Maxwell distributions:
|
|
|
(1.7)
|
Converting equation (1.7)
to average energy terms <E>=m<vx2> we have:
|
|
|
(1.8)
|
Now vpdt=δxp so we have the following
differential equation which can only be solved iteratively:
|
|
|
(1.9)
|
It turns out the that exponential value for the piston
speeds used in the program is very close to 1.0 so the program uses the value
|
|
|
(1.10)
|
Appendix: Rate of wall hits
Assuming that the container dimensions are much greater that
the mean free path, l, we have the
following cases:
1. If the distance of
the atom from the wall is equal to l,
then the probability that the atom hits the wall in l/vx seconds is 0.5.
2. If the distance of
the atom from the wall is equal to fl,
then the probability that the atom hits the wall in l/vx seconds is 0.5/f2.
There are N(fl/xP) atoms within nl of the piston.
Converting statement
2 into an equation we have:
|
|
|
(1.11)
|
The number of atoms at distance |xp-x| hitting
the wall per second is then n(x)vx|x-xp|/vx.
|
|
|
(1.12)
|
The mean free path is
|
|
|
(1.13)
|
where a is the radius of the hard disc atom.
Then equation (1.12)
becomes:
|
|
|
(1.14)
|
Now we must integrate this expression over all the x's from
0 to xp.
|
|
|
(1.15)
|
