Gas Compression through A Nozzle
Introduction
This animation shows the periodic compression and expansion
of a gas by a moving piston. The original idea here was to show how a wind is
generated by a gradient in atomic density.
An extreme gradient is generated here by having a nozzle at the right
side of the cylinder and having an almost perfect vacuum beyond the nozzle.
In order to show significant results, we will permit piston
speeds that are approximately equal to the average thermal speeds of the atoms.
This will show how interesting atomic density distributions can build up on the
face of the advancing piston. What is
interesting is that we can get reasonably good agreement for energy, force on
piston, and average atomic speed between the hard sphere kinetic calculations
and first principles results derived from classical gas pressure theory
analysis. This agreement exists even
though the gas is never in a quasi-static state.
Figures
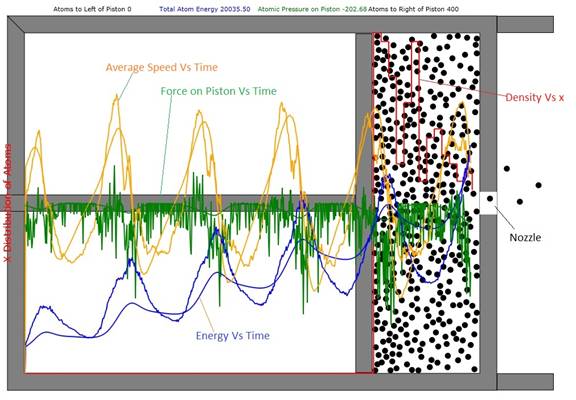
Figure 1: Cylinder and Piston filled with 400
atoms. The total atom energy has increased from about
3500 to 20036 units. By viewing the red
histogram, one can see that the density of the atoms to the right of the
right-going piston is highest. This is
because piston speed slightly exceeds average atomic speed so the atoms can't
diffuse away fast enough. The smooth blue curve is the first-principles result
for the total atom energy and the smooth green curve is the first principles
result for the force on the piston. The
orange curves give the average velocity in the horizontal direction.
Calculation of Energy during adiabatic compression
Adiabatic
compression means that no heat flows into the system under observation during
that compression. Atoms do gain kinetic energy (the temperature
increases) during adiabatic compression because of the mechanical energy
provided by the moving piston. The
reason is that a atom with x velocity component -vx (where vx>0
for the time being) that collides with the front side of a piston moving in the
x direction at speed vp>0 rebounds with an x component of speed vx+vp
i.e.
and therefore the colliding atom gains energy.
We will use
the term "front side" to describe the face of the piston whose
outward normal is parallel with vP and the term "back
side" to describe the face of the
piston whose outward normal is anti-parallel with vP.
One might
object that the opposite speed change happens when an atom hits the back side
of the piston and that is absolutely correct.
However, as the animation shows, there is often a much higher density atoms
on the front side of the piston than the back side and therefore there is a net
energy gain per cycle.
In the
following, we will consider only the motion of the piston in the positive x
direction. By symmetry, the results for
energy transfer for motion in the negative x direction must be the same. Also we set the piston coordinate, xP,
to zero when the piston is at the center of the cylinder.
The average time between collisions of the atom with the
front of the moving piston is
and the time between collisions with the back of the moving
piston is
where L is the total length of the cylinder and <vx>
is the average x component of the velocity.
where Etotal is the total kinetic energy of the
atoms and N is the total number of atoms.
The total rate of collision on the front and back side is then
just the number of atoms, N, on that side divided by its average interval
between collisions:
The energy transfer per collision is just due to the added
or reduced x component of velocity due to the moving piston:
where m is the mass of the atom and vp can be
positive or negative.
Then the rate of energy added per second to atoms colliding
with the front of the piston is :
and the rate of energy subtracted per second from atoms
colliding with the back of the piston is:
So the program continuously integrates the dE/dt increments
to estimate the added kinetic energy associated with the periodic adiabatic
compressions. Of course here, xp and
vp vary sinusoidally with time.
Calculation of Force on Piston
The net
force on the moving piston due to atoms hitting it is calculated in an entirely
similar way to the energy induced in the atomic gas by the piston. First we have the rates of atoms hitting the
front and back of the piston:
The momentum imparted to the moving piston for each
collision averages:
And therefore the total momentum in the positive x direction
imparted per second from all the atoms hitting the front and back of the piston
is:
and therefore the total force on the piston is:
Obviously, if NF=NB we have a force on
the piston even when vp=0.
That is reasonable, since, when, for example, xP>0, the
gas on the right side is compressed.
Calculation of Average Atomic Horizontal Speed
Again, we
have the average rate of collisions with the moving piston:
and with each collision we expect the atom to gain speed vp. Then the added speed in +x direction would
be:
where both the numerator and denominator vary sinusoidally.
