2D Molecular Energy
Distributions Animation
Introduction
This
animation demonstrates the dependence of 2D molecular energy distributions on
vibration mode excitation as well as linear translation and rotation. The results are interpreted by both
histograms of the frequency distributions of energies and algebraic plots
of the expected distributions.
Figures:
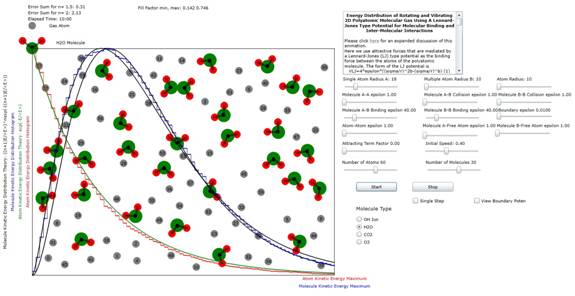
Figure 1: Plot
of atomic and molecular distribution of molecule H2O (water). The results (n=1.5) indicate that only two of
the possible 3 modes of planar vibration
are excited at these energy levels.
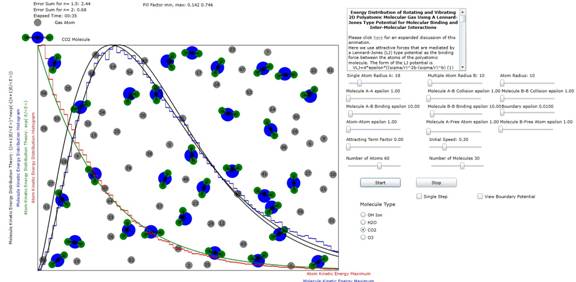
Figure 2: Plot
of atomic and molecular distribution of molecule CO2 (carbon dioxide). The results (n=2) indicate that all of the possible 3 modes of planar vibration are excited at these energy levels.
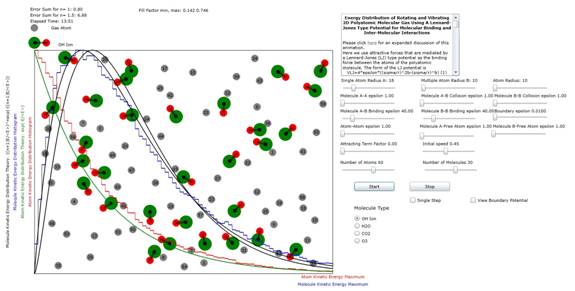
Figure 3: Plot
of atomic and molecular distribution of the OH ion (hydroxide). The results (n=1) indicate that single mode of planar vibration is marginally excited at these energy levels.
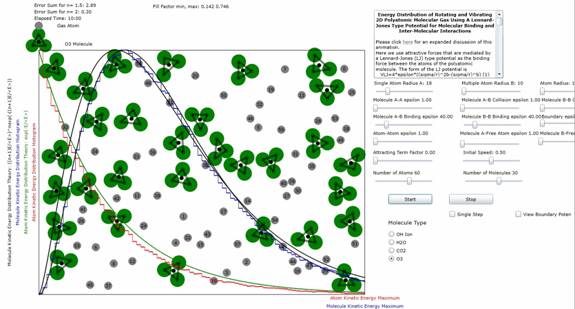
Figure 4: Plot
of atomic and molecular distribution of molecule O3 (ozone). The results (n=2) indicate that all 3 of the possible 3 modes of planar
vibration are excited at these energy
levels.
Analysis
For each degree of freedom, the multiplier of the fraction
in the exponential increases by 1/2 while the power of the multiplier in the
expression for energy distribution increases by 1/2. Then for 2n+2 degrees of freedom, the
expression for distribution of energies is
|
|
|
(1.1)
|
where T is the total kinetic energy of the molecule.
If we want to convert this to a probability we must set the
integral from 0 to infinity of f(E) to 1:
|
|
|
(1.2)
|
The integral in the denominator is fairly simple letting :
|
|
|
(1.3)
|
and therefore
|
|
|
(1.4)
|
|
|
|
(1.5)
|



