The Photoelectric Effect
Introduction
The photoelectric (PE) effect was the second big pillar that supported the quantum theory that had been initiated by Max Planck in 1901 to explain black body radiation. The PE effect data that had been obtained experimentally was explained by a surprisingly simple theory for which Albert Einstein received the Nobel prize. It showed that electrons were emitted from a photocathode individually and with kinetic energy that depended on the wavelength of the light incident on the cathode.
Experiment and Theory
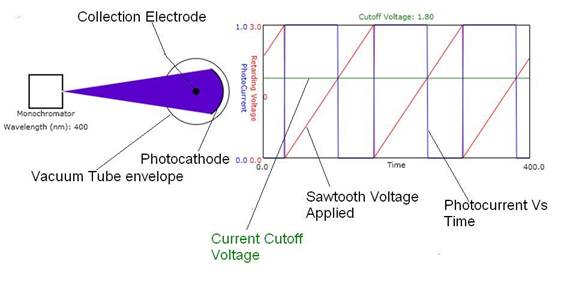
Figure 1: Experimental setup for the PE effect. The Collection Electrode (CE) has a retarding
(negative) voltage applied to it while the Photocathode is kept at ground
potential. The current due to emitted
photo-electrons that have enough kinetic energy to reach the CE is applied to
one channel (blue) of an oscilloscope shown at the right. The retarding voltage applied to the CE is
applied to the other channel (red) of the oscilloscope. The cutoff voltage is
indicated by the horizontal green line that moves to lower voltages as the
monochromator wavelength is increased.
To avoid a lot of other difficulties, I elected to use the oscilloscope on the right as a readout for the PE effect. The equation for the electron’s kinetic energy at the CE is:
![]()
where h is Planck’s constant, c is the speed of
light, l is the wavelength of the
incident light, e is the charge on the electron and V is the retarding
voltage. As a reference, for l=400 nm, hc/l=5x10-19
Joules while, for V=1 volt, eV=1.6x10-19 Joules. Thus it will require about 3 volts to
completely cut off the current when l=400
nm.