Sublimation and Deposition
Introduction
Sublimation and Deposition are complex molecular phenomena. A good animation of these reversible processes is essential to their understanding. The animation discussed here will provide that understanding.
Figures
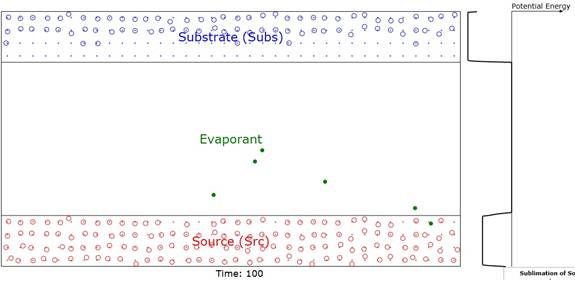
Figure 1: Showing surface barriers (potentials) and
liquid, gas and solid phases color coded to be red, green, and blue. Note that
some of the red molecules have evaporated (shown as green) into the space
between the Source and Substrate domains and are
therefore candidates for deposition on the latter.
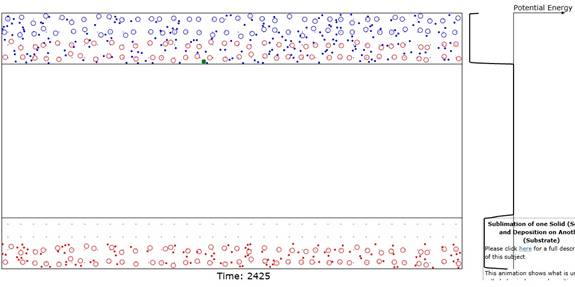
Figure 2: Showing result when coating (bottom 2 red
layers) on Substrate is complete. Note
that the top 2 Source layers are gone and only the red lattice anchor sites of
these rows are visible in this Figure.
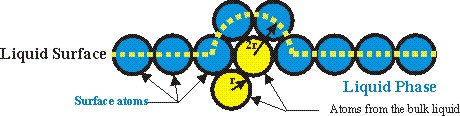
Figure 3. Figure related to Reference 1 showing the the
heat of vaporization is a fairly simple function of the product of the surface
tension and the area of a displaced hemisphere through which the centers of
atoms pass at the surface of a liquid or solid.
Physics of Evaporation and Condensation[1]
All atoms or molecules have critical energies, Ee, above which they can exit the liquid or solid domain in which they are presently bound and enter the gas phase (evaporate) outside that domain. As shown in Reference 1, this energy is directly proportional to the surface tension (or, equivalently, the surface energy) of the surface of the particular liquid or solid in question (see Figure 3). Similarly all atoms or molecules in a gaseous state have critical energies, Ec, below which they may condense or dissolve into a neighboring liquid or solid surface or volume. Gaseous atoms with energy higher than Ec just reflect off the interface between the media. Since total energy in a closed system is conserved, the energy that a atom loses evaporating from the liquid or solid state is regained when it is condensed back into the liquid or solid state. In physics we call the energy needed for evaporation the potential barrier energy. An atom that has just been evaporated loses the potential barrier energy which is a substantial fraction of its kinetic energy while an atom that has just been condensed gains back a similar kinetic energy. So recently condensed atoms are "hot" but that extra kinetic energy is quickly dissipated via scattering with the cooler atoms of the liquid or solid. The animation permits adjustment of the potential energies as well as the average energies of the liquid, gas, and solid domains.
Evaporative Cooling
It's not so obvious from what has just been discussed, but when an atom has enough energy to evaporate, it carries a substantial fraction of its native media's energy with it. Since the atomic kinetic energy distribution is an exponential
![]()
and the total energy greater than Ec
is given by ratio of the integrals:
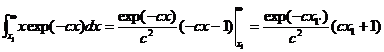
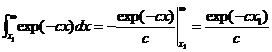
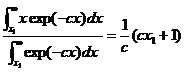
where c=1/kT and Ec =x1. Note that the average energy of the entire
energy range is kT.
Then only those atoms with E>Ec can evaporate, the change in average energy is:

which is essentially the average energy of all the atoms with energy above Ed. Thus, on average, when an atom evaporates, it reduces the energy of the entire atomic ensemble by:
![]()
The total loss of energy when a large group, Ne, of atoms evaporates is of course
![]()
Solving for the final temperature, Tf, and taking the differential we have
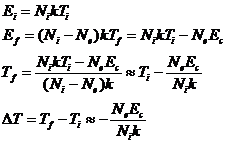
where the approximation is valid
when Ni>>Ne.
Another way of expressing this equation is to compute the heat energy of evaporation, Q, per gram molecular weight, n, evaporated:
![]()
where A is Avogadro's number.
Specifics of Sublimation and Deposition
In
this discussion, the Source and Substrate solids are made up what will be
called ions and electrons. The ions
vibrate around fixed uniformly spaced anchor points in the solids. The electrons are free to range over the
entire domain of the solid and they regularly collide with the ions, exchanging
energy with them. Since the electrons
receive some energy from one ion and give some energy to another ion, this
provides a way of coupling the ions.
The
ions have to have more than a certain critical energy climb the potential hill at
the surface of the solid and thereby to escape their host and become vapor
atoms. This is called the enthalpy of
evaporation. On the other hand, one
might think that
vapor atoms would always be deposited on the subs since they only have to drop
down the potential hill at its surface.
That concept, however, does not take into account the fact that the
vapor atom undergoes collisions with the atoms at the surface of the Substrate. If the kinetic energy of the vapor atoms is
too large and/or the direction of the nearest subs ion is outward from the
surface, the vapor atoms just recoil from the surface rather than being
deposited on it. This process has the
effect of compacting the coating which is usually a good thing but does not
further increase the thickness of the coating.
None of these recoils are illustrated since they're beyond the scope of
this animation but the viewer needs to understand that they happen on regular
basis.