Thermal Conductivity and the Evolution of the Second Law of
Thermodynamics
Introduction
This
animation shows the exchange of energy of three adjacent gas domains at the two
impermeable boundaries between them. The
impermeable boundaries are needed in order to keep the atoms from the warmer
region from simply diffusing into the cooler region. So, in effect, our three regions are solids
that can exchange energy with the adjacent region but don't lose any of their
atoms to that region.
In a
previous animation we showed that the more energetic gas atoms, on average,
give up during collisions, some of their energy to the less energetic gas
atoms. That is exactly what happens at
the boundary between two gas domains where the respective gases have different
energies (or, equivalently, different temperatures). This results in heat energy flow from the
higher temperature domain to the lower temperature domain. It is shown in the
Appendix that the flow of energy from more energetic particles to less
energetic particles occurs even for particles of different mass. This
is a statement of the second law of thermodynamics for microscopic bodies. When I
apply the same principles to whole regions of higher energy (temperature) then
this shows the evolution of the second law of thermodynamics for macroscopic
bodies as advertised in the title.
In this
document, I will first give the standard thermal conductivity treatment for the
three slabs of which this system is comprised.
Then I will document on a more microscopic basis the dynamics how the time-dependent
temperature distributions are achieved.
In the Appendix I show the mathematics of the velocity changes when two
particles of different mass and arbitrary velocities collide.
Figures
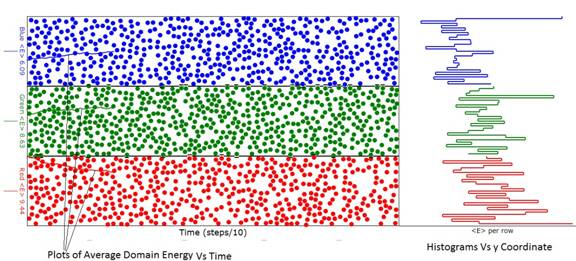
Figure 1: Diagram of the animation considerably before
energy (temperature) evolution was complete.
Note that the average energy (temperature) of the red domain exceeds
that of the green domain and the green domain average energy exceeds that of
the blue as can be seen from the ordinate labels at the left of the domains. In this animation the green domain had 600
atoms while the red and blue domains had 500 atoms. If you look closely, you will see that the
boundaries between the domains are "puckered" when one of the atoms
of a domain transgresses into the other domain.
Sometimes when this happens, the transgressing atom collides with an
atom in the other domain exchanging energy with it. That is the essence of how heat is exchanged
between domains.
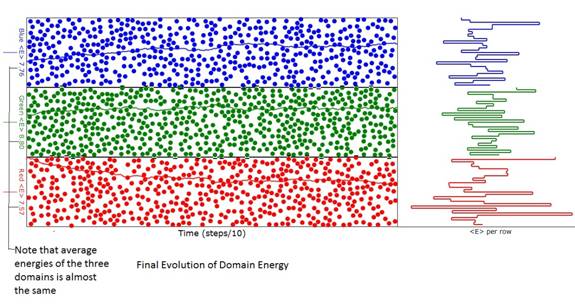
Figure 2: Diagram of the animation when energy
(temperature) evolution was reasonably complete. Note that the average energy (temperature) of
the three domains is almost the same as can be seen from the ordinate labels at
the left of the domains. The differences
are due to the finite number of atoms.
Animation Setup
For
simplicity I have chosen to have just three domains arranged as layers. The top and bottom layer can be considered to
represent solids. In solids, quantum
entities known as phonons carry heat via vibrational modes. Phonons behave much
the same as the kinetic energies of atoms so their equilibration and exchange
of energies is reasonably represented by the kinetic energy of atoms. For even more simplicity I have chosen the
number of atoms in the top and bottom layers to be the same so, when their
average energies (temperatures) are equal, the thermal conductivity of these
layers will be the same. To make the
problem more interesting, the learner may select (within limits) the number of
atoms in the middle layer and this will make the thermal conductivity (and heat
capacity) of that layer different from the other two.
Macroscopic Temperature Calculations
To compute
the temperature distributions Vs time we need to have access to two parameters:
1. The thermal conductivity, K (Watts
m-1 Kelvin-1)
2. The heat capacity, C (Joules m-3 Kelvin-1)
The ratio of K to C is called the diffusivity, D=K/C (m2/sec).
The temperature, T, Vs time, t, can be found from the
differential equation:
(1)
where the first inverted delta on the left is the divergence
and the second is the gradient.
Initial Conditions Matching with Complementary
Solution
We need a
solution to specify the transients that occur starting at t=0. For that we use a technique called "separation
of variables" which I will explain next.
We have the three equations:
(2)
We make the assumption that each of the Ts can be written:
Then equation 2 can be rewritten:
(3a,b)
where Λ is called the eigenvalue.
The solution for equation 3b
(4)
Describing the Initial Conditions
We have
three layers of thickness L1, L2, and L3 and
temperatures T1, T2, and T3.
The initial temperature, Ti, Vs y can be written
piecewise (denoted by subscript p) as:
(5)
Similarly the diffusion coefficient D can be expressed piecewise
as:
(6)
In order to solve the differential equation we need to express
Tp and Dp as periodic on the interval P=L1+L2+L3
where P is the period of what will be the Fourier series describing T(y) and
D(y). The series expressing Ti is:
where
It is quite easy to perform the integral for gn:
Note that the average value of T is equal to g0
Equations 5 and 6 have the problem that their first and
second derivatives with respect to y are infinite at the interfaces between the
layers. That can be rectified by using a
"soft" step function at the interfaces. This step function is implemented by using
1/2 period of a sine wave.
where w is 1/2 of the width of the step, y0 is
the center of the step, Tavg=(Ta+Tb)/2, and Tdiff=(Tb-Ta)/2
where Ta is the temperature at the start of the step and Tb
is the temperature at the end of the step.
Similar expressions can apply for steps in D. The step function must be
applied at the transition between T1 and T2, T2
and T3 and for the transition from T3 back to T1. This results in a more complicated function Tp(y)
and it is necessary to do the integrations for gn numerically.
Case 1:D is the same in all layers:
The second derivative of Ti with respect to y is:
If D is the same in the 3 layers then we can immediately
write that
so that
The results for T Vs t at various y values are shown in
Figure 3 below:
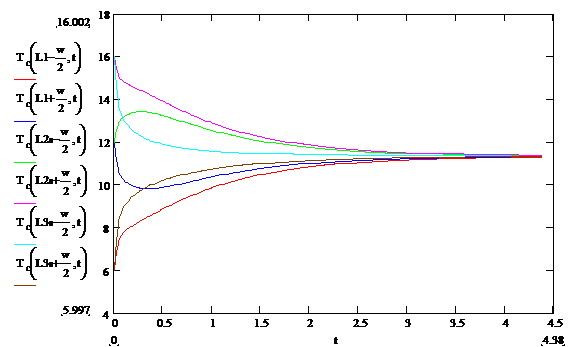
Figure 3: Temperature Vs time at several locations
near the temperature transition boundaries computed using alternative software. Note the very high rates of change of
temperatures near t=0. Note that the
second and third curves are not even monotonic in their progress toward their
final value. This is due to the very large gradient at the zone
boundaries. This result is in good
qualitative agreement with a similar finite element analysis.
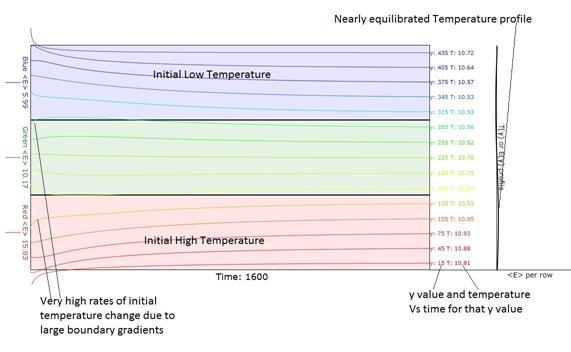
Figure 4. Temperature Vs Time obtained when the
"Macroscopic" button is pressed.
Note the very high rates of initial temperature change due to large
initial boundary gradients. The 15
traces are displaced so that their final )equilibrated temperature ordinates
lie at the y value for which that trace stands.
In the green region, note that the slope of the temperature Vs time
actually changes sign as it progresses toward equilibrium. That is due to the fact that it is initially
adjacent to a much cooler or hotter region.
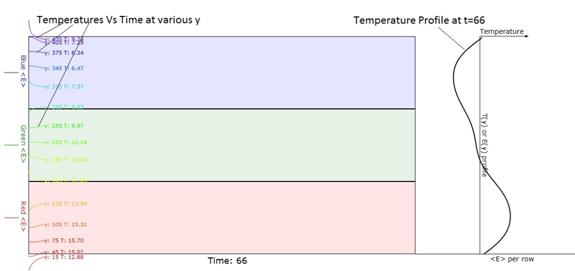
Figure 5. Earlier version of Figure 4. This shows a better description of the
temperature profile at the right.
Case 2: D is different in all layers
In exactly the same manner we can write a Fourier series to
express Dp:
It is important to know that our simple
tri-level functions are adequately expressed for absolute values of n that are
less than 50.
The first derivative of Ti (y) including the D
term in equation 2 is:
The final y derivative in equation 2 results in:
This equation shows that a complicated function of y (the f
terms) has taken the place of the previous constant eigenvalue. We could use perturbation theory to determine
the effects of the variation of D on the original eigenfunctions. But that will be beyond the scope of this
document.
Energy Distribution of a Gas
1. Standard Thermal Physics Treatment
The
Boltzmann probability law for particle height, in a gravity (or acceleration
field), g, is:
(1)
where N0 is a normalization constant, m is the
mass of the atom, k is Boltzmann's constant, v is the speed of the atom, and T
is absolute temperature. It is easy to
show that the average value of E, <E>, or mv2/2 is equal to kT
using the integral:
Appendix
Particle-Particle Collisions of Different Mass
and Velocity
The
following will prove that, via particle-particle collisions, energy always
flows from more energetic particles to less energetic particles. In order to have heat flow (thermal
conductivity) from hotter (more energetic) regions to colder (less energetic)
regions it is necessary that the previous statement be true.
Here we
will consider spherical particles which have the different masses, m1
and m2, and diameters, D1 and D2. The centers of the spheres will be labeled (x1,y1,z1)
and (x2,y2,z2). Upon collision, the momentum transferred
between the spheres will always be along the unit vector:
(2)
where
is the distance between centers. Since the animation is illustrated in only 2
dimensions, the collision analysis will assume a containing box that is large
in the x and y dimensions but very thin in the z dimension.
The expression for the final momenta in terms of the initial
momenta is:
(3)
where the apostrophe on the left side of the equations
indicates the final velocities. We know
that the energies are conserved so
(4)
The directions of the change in momenta are along the vector
of centers, u, and the values of the
changes of momenta must be equal and opposite.
(5)
where M has units
of mass and is still to be determined.
Then
(6)
Now we can use equation 6 in equation 4 to solve for the
value of Mδv.
(7)
where the large dot stands for the dot product and equation 7
simplifies to:
(8)
We can now make the identification:
(9)
where M is known
as the "reduced mass".
Equations 6 and 8 are a complete solution for the final momenta.
The final velocities are computed by dividing both sides of equations 6 by
their respective masses:
(10)
Suppose m2>m1. Then we see that the magnitude of the speed
added to molecule 1 will be larger than the magnitude of the speed removed from molecule 2. We can easily see from equation 7 that, even though the averages of the dot
products are zero, that the collision
results in an increased kinetic energy for molecule 1 and a decreased kinetic
energy for molecule 2 because of the mass term in the denominators. Rewriting equation 4 we have:
(11)
If the initial speeds are the same then the only difference
between E1 and E2 is due to the M2 term in the
kinetic energies.
(same speeds)
If the speeds are different, say v2>v1,
but the masses are the same, then the difference in kinetic energies is due to
the Mm term in the kinetic energies:
The above equation can be re-written:
where
is the cosine of the angle between vi and u. The average value over all possible angles
between vi and u of the cosine squared terms is 1/2 so
we can write
(same
masses)
Therefore, averaged over all possible angles between u and v,
the change of kinetic energy for particle 1 will be positive since v2>v1.
This degradation of the energy of the larger mass or higher
speed atoms and/or speed leads to what is called the zeroth law of
thermodynamics-all energy distributions tend to be become equalized. An example of this would be two gases of
different molecular masses as well as different temperatures injected into an
insulated box. The gas temperatures (or
average energies) will equalize leaving the average speed of the gas with more
massive molecules less than that of the gas with less massive molecules but the
energies will tend to become equal due to the energy Vs velocity law we just
proved.
Again rewriting equation 4 we have:
(12)
Let's compute the E1 and E2 for the
general case where both m and v are different.
(13)
Inserting the expression for Mδv
we have:
(14)
Rewriting equation 14 where I've explicitly given the
expression for δv
we have:
where v1 and v2 with the carats over
them are unit vectors in the direction of v1
and v2,
respectively. Their dot products with u are just the cosine of the angle
between them and u and will be
denoted c1 and c2.
(15)
It's clear
that, over many collisions, the c1c2 terms average to
zero.
Then the following expression for the average change of the energies
per collision will be valid
(16)
Note that
(17)
(18)
The average value of c12 and c22
over very many collisions is 1/2 so we can rewrite this as
(19)
and then recognizing that the quantities in brackets are the
original energies we have
(20)
From this equation we can say:
1. If E2>E1 the energy of particle
1 increases by a factor proportional to the difference.
2. If E1>E2 the energy of particle
2 increases by a factor proportional to the difference.
We may also subtract the above equations to write the
differential equation:
(21)
where dn is the increment in the number of collisions and
the equation has the solution:
(22)
where (E1 -
E2)0 is the initial value of the energy difference. The above equation shows that the energy
difference between atoms 1 and 2 will degrade to zero as the number of
collisions, n, increase. This result is
for only one pair of atoms that interact by atom-atom collisions in a box. But this
results also extends to the energy distribution of many atoms of
possibly different masses colliding inside a box.
Removing Particle Overlap
Since the
animation is digital we can expect that most often the collision condition will
be realized when the distance between the centers of the two spheres,r12,
is less than D=(D1+D2)/2.
To handle this I will increase the distance between the centers by the
difference
by shifting each sphere in opposite directions by dr/2 along
u.
Thus we will move the centers of each particle by the vectors:
(23)




