Animation of an Electric Gas Discharge
Introduction
An electric gas discharge (sometimes called a plasma or arc) requires two ingredients:
1. A gas whose particles can be ionized (charged) by either collisions or ionizing radiation like photons.
2. An electric field to accelerate the ionized particles.
Although it is possible to have a gas discharge (e.g. lightning) in open spaces, the discharge is more controllable when the gas is in an insulated chamber and the electric field is produced by positive and negative electrodes at the two ends of the chamber. The chamber can also be partially evacuated which enhances the rate of energy gain by the ions and their electrons.
The gas discharge that we will animate here will be a chaotic process where the number of ions will suddenly increase exponentially when the average particle energy reaches the value where the ion yield per ion created exceeds 1.0. "Stable" gas discharges are possible in the laboratory but these involve feedback from the electric power supply and are too difficult to reliably animate.
Figures
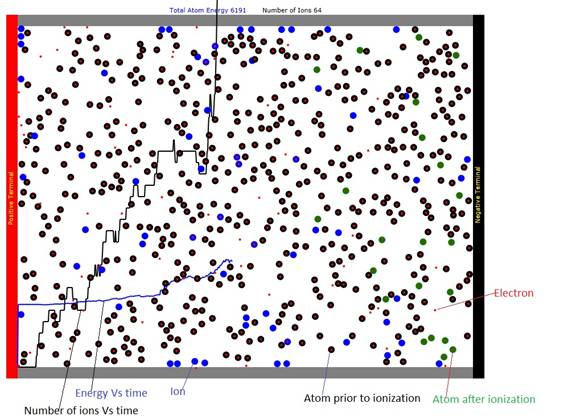
Figure 1: Gas discharge chamber with positive electrode at left and negative electrode at right. The positive ions drift to the right and the negative electrons drift to the left.
Calculations and Comments
The gas
atoms have an ionization energy. When
the ionization energy is reached and an atom collides with another atom, one of
the atoms will usually be ionized creating an ion and an electron. The charged particles are accelerated by the
electric field created by the electrodes.
The time-averaged effect of this acceleration is that the charged
particles gain energy since
where m is the particle mass, vx is its speed in the x direction, t is time, e is the charge of the particle and E is the electric field assumed to be along the x direction.
When the charged particles gain enough energy that their total kinetic energy exceeds the ionization energy of the neutral atoms, they can cause the atoms they collide with to become ions (colored blue) and this process can cause the number of ions to grow exponentially resulting in what is called an arc. An alternative fate of the positive ions is that they can collide with the negative electrode and attach an electron from it and become neutral. Atoms that have become ions and then are discharged by the negative electrode are shown as green in Figure 1 and in the animation.
We can easily show that the thermal energy of atoms at room temperature (or even at the temperature of the sun, 6000 Celsius) is not large enough to create a significant fraction of ions. However, we always have a significant number of high energy (e.g. cosmic ray) photons that can create ions and the animation uses these photons to initiate the energy gain process.
There are three distinct particle types, atoms, ions, and electrons. These particle types give rise to many different types of particle-particle collision:
1. Atom-Atom
2. Atom-Ion
3. Atom-Electron
4. Ion-Ion
5. Ion-Electron
6. Electron-Electron
The basic units of the animation are the ion and the electron and the atom is portrayed as an ion with a red electron at its center. The mass of the Atom is the sum of the masses of the Ion and electron and in collisions between Atoms the electron's velocities follow those of the Atom. The kinetics of particle-particle collisions are adequately explained by the following reference:
There are three types of particle-boundary collision:
1. Atom-boundary collision which just inverts the normal component of velocity of both the electron and ion of the atom.
2. Ion-Boundary collision which inverts the normal component of velocity of the ion. As a special case, after an ion collides with the negative electrode, an electron adopts the ion's location and velocity and it becomes a neutral atom.
3. Electron-Boundary collision which just inverts the normal component of velocity of the electron.